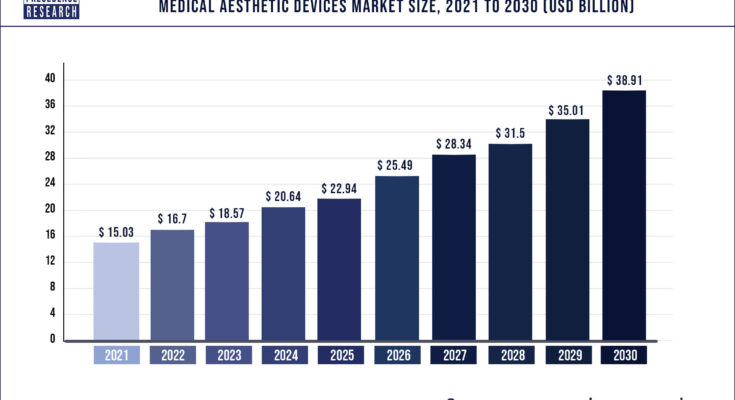
A report from Precedence Research projects the medical aesthetic devices market size will reach US$ 38.91 billion by 2030, up from US$16.7 billion in 2022.
An increase in demand for minimally invasive procedures, surging prevalence of congenital teeth, rising incidences of face deformities, technological advancements in aesthetic devices, and a rise in the number of cosmetic surgeries are the prominent factors that are boosting the global aesthetic devices market growth. The report contains 150+ pages of detailed analysis. The base year for the study has been considered 2021, the historic year 2017 and 2020, and the forecast period considered is from 2022 to 2030.
Download Free Sample Copy with TOC, Graphs & List of Figures @ https://www.precedenceresearch.com/sample/1773
Some of the key players in the global medical aesthetic devices market include:
- Allergan plc
- Dentsply Sirona Inc.
- Johnson & Johnson
- LUMENIS LTD
- MERZ PHARMA GMBH & CO. KGAA
- Hologic, Inc.
- Sientra Inc
- SYNERON MEDICAL LTD
- VALEANT PHARMACEUTICAL INTERNATIONAL, INC
- Zimmer Biomet Holdings, Inc.
Covid-19 Scenario-
- The global economic recession, decline in product demand, and temporary closure of most of the beauty centers affected the medical aesthetic devices market, especially during the initial phase of the pandemic.
- However, the market is anticipated to recoup really soon.
Report Scope of the Medical Aesthetic Devices Market
| Report Coverage | Details |
| Market Size by 2030 | USD 38.91 Billion |
| Growth Rate from 2022 to 2030 | CAGR of 10.7% |
| Largest Market | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Type, Application, End User, Region |
The surgical segment to dominate by 2030
By application, the surgical segment contributed to the lion’s share in 2021, holding more than two-thirds of the global medical aesthetic devices market. This is attributed to the fact that surgical medical aesthetic procedures are more accessible and affordable when compared with non-surgical measures. However, the non-surgical segment is projected to manifest the fastest CAGR of more than 11% from 2022 to 2030.
Market Segmentation
By Type
- Devices
- Energy-based Aesthetic Device
- Laser-based Aesthetic Device
- Radiofrequency (RF)-based Aesthetic Device
- Light-based Aesthetic Device
- Ultrasound Aesthetic Device
- Non-energy-based Aesthetic Device
- Botulinum Toxin
- Dermal Fillers and Aesthetic Threads
- Microdermabrasion
- Other
- Energy-based Aesthetic Device
- Aesthetic Implants
- Facial Implants
- Breast Implants
- Others
By Application
- Surgical
- Non Surgical
By End User
- Hospitals and Clinics
- Medical Spas and Beauty Centers
North America held the major share in 2021
By region, North America dominated in 2021, garnering nearly two-fifths of the global medical aesthetic devices market, owing to increased adoption of medial aesthetics, enhanced technological advancements, and development of novel products by the key players in the province. The market across Asia-Pacific, simultaneously, is expected to cite the fastest CAGR of more than 11% throughout the forecast period. Improvement in R&D facilities, an increase in medical tourism, and a rise in awareness about medical aesthetics drive the market growth in the region.
Regional Segmentation
- Asia-Pacific [China, Southeast Asia, India, Japan, Korea, Western Asia]
- Europe [Germany, UK, France, Italy, Russia, Spain, Netherlands, Turkey, Switzerland]
- North America [United States, Canada, Mexico]
- South America [Brazil, Argentina, Columbia, Chile, Peru]
- Middle East & Africa [GCC, North Africa, South Africa]
Research Methodology
A unique research methodology has been utilized to conduct comprehensive research on the growth of the global medical aesthetic devices market and arrive at conclusions on the future growth prospects of the market. This research methodology is a combination of primary and secondary research, which helps analysts warrant the accuracy and reliability of the draw conclusions. Secondary sources referred to by analysts during the production of the global market report include statistics from company annual reports, SEC filings, company websites, World Bank database, investor presentations, regulatory databases, government publications, and industry white papers. Analysts have also interviewed senior managers, product portfolio managers, CEOs, VPs, and market intelligence managers, who contributed to the production of our study on the market as a primary source.
These primary and secondary sources provided exclusive information during interviews, which serves as validation from mattress topper industry leaders. Access to an extensive internal repository and external proprietary databases allows this report to address specific details and questions about the global medical aesthetic devices market with accuracy. The study also uses the top-down approach to assess the numbers for each segment and the bottom-up approach to counter-validate them. This has helped to estimate the future prospects of the global market more reliable and accurate.
Why should you invest in this report?
If you are aiming to enter the global medical aesthetic devices market, this report is a comprehensive guide that provides crystal clear insights into this niche market. All the major application areas for medical aesthetic devices are covered in this report and information is given on the important regions of the world where this market is likely to boom during the forecast period of 2022-2030 so that you can plan your strategies to enter this market accordingly.
Besides, through this report, you can have a complete grasp of the level of competition you will be facing in this hugely competitive market and if you are an established player in this market already, this report will help you gauge the strategies that your competitors have adopted to stay as market leaders in this market. For new entrants to this market, the voluminous data provided in this report is invaluable.
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Medical Aesthetic Devices Market
5.1. COVID-19 Landscape: Medical Aesthetic Devices Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Medical Aesthetic Devices Market, By Type
8.1. Medical Aesthetic Devices Market, by Type, 2022-2030
8.1.1 Devices
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Aesthetic Implants
8.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Medical Aesthetic Devices Market, By Application
9.1. Medical Aesthetic Devices Market, by Application, 2022-2030
9.1.1. Surgical
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Non Surgical
9.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Medical Aesthetic Devices Market, By End User
10.1. Medical Aesthetic Devices Market, by End User, 2022-2030
10.1.1. Hospitals and Clinics
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Medical Spas and Beauty Centers
10.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Medical Aesthetic Devices Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Type (2017-2030)
11.1.2. Market Revenue and Forecast, by Application (2017-2030)
11.1.3. Market Revenue and Forecast, by End User (2017-2030)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Type (2017-2030)
11.1.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.1.4.3. Market Revenue and Forecast, by End User (2017-2030)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Type (2017-2030)
11.1.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.1.5.3. Market Revenue and Forecast, by End User (2017-2030)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Type (2017-2030)
11.2.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.3. Market Revenue and Forecast, by End User (2017-2030)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Type (2017-2030)
11.2.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.4.3. Market Revenue and Forecast, by End User (2017-2030)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Type (2017-2030)
11.2.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.5.3. Market Revenue and Forecast, by End User (2017-2030)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Type (2017-2030)
11.2.6.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.6.3. Market Revenue and Forecast, by End User (2017-2030)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Type (2017-2030)
11.2.7.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.7.3. Market Revenue and Forecast, by End User (2017-2030)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Type (2017-2030)
11.3.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.3. Market Revenue and Forecast, by End User (2017-2030)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Type (2017-2030)
11.3.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.4.3. Market Revenue and Forecast, by End User (2017-2030)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Type (2017-2030)
11.3.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.5.3. Market Revenue and Forecast, by End User (2017-2030)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Type (2017-2030)
11.3.6.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.6.3. Market Revenue and Forecast, by End User (2017-2030)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Type (2017-2030)
11.3.7.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.7.3. Market Revenue and Forecast, by End User (2017-2030)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Type (2017-2030)
11.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.3. Market Revenue and Forecast, by End User (2017-2030)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Type (2017-2030)
11.4.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.4.3. Market Revenue and Forecast, by End User (2017-2030)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Type (2017-2030)
11.4.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.5.3. Market Revenue and Forecast, by End User (2017-2030)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Type (2017-2030)
11.4.6.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.6.3. Market Revenue and Forecast, by End User (2017-2030)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Type (2017-2030)
11.4.7.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.7.3. Market Revenue and Forecast, by End User (2017-2030)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Type (2017-2030)
11.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.5.3. Market Revenue and Forecast, by End User (2017-2030)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Type (2017-2030)
11.5.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.5.4.3. Market Revenue and Forecast, by End User (2017-2030)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Type (2017-2030)
11.5.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.5.5.3. Market Revenue and Forecast, by End User (2017-2030)
Chapter 12. Company Profiles
12.1. Allergan plc
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. DentsplySirona Inc.
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Johnson & Johnson
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. LUMENIS LTD
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. MERZ PHARMA GMBH & CO. KGAA
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Hologic, Inc.
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. SientraInc
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. SYNERON MEDICAL LTD
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. VALEANT PHARMACEUTICAL INTERNATIONAL, INC
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Zimmer Biomet Holdings, Inc.
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
Purchase Full Research Report (Single User License US$ 4500) @ https://www.precedenceresearch.com/checkout/1773
Contact Us:
Precedence Research
Call: +1 9197 992 333
Email: [email protected]
Address: Apt 1408 1785 Riverside Drive Ottawa, ON, K1G 3T7, Canada