This report aims to provide detailed insights into the global companion diagnostic market. It provides valuable information on the type, procedure, application, and region in the market. Furthermore, the information for these segments, by region, is also presented in this report. Leading players in the market are profiled to study their product offerings and understand the strategies undertaken by them to be competitive in this market.
Key Factors Driving Market Growth:
The growth of the Companion Diagnostics Market is tied primarily to the advantages of companion diagnostics, the growing need for targeted therapy, the increasing importance of personalized medicine, the rising global incidence of cancer, and the ever-increasing application areas of companion diagnostics. The increasing demand for next-generation sequencing, the growing significance of companion diagnostics in drug development, and the rising number of clinical trials are also expected to present growth opportunities for players in the market, which also expected to support market growth in the coming years.
Expected Revenue Growth:
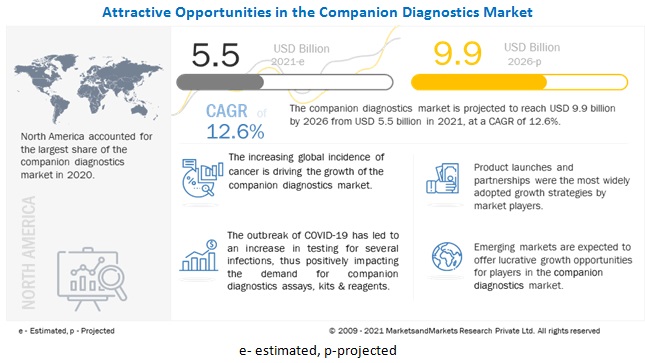
[331 Pages Report] The global Companion Diagnostics Market is projected to reach USD 9.9 billion by 2026 from USD 5.5 billion in 2021, at a CAGR of 12.6%
Download PDF Brochure:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=155571681
Growing Need for Targeted Medicines
With advances in genetic sequencing and genomics, it is now widely believed that drugs can show varying outcomes in different individuals. A better understanding of the genetic characteristics or biomarkers of an individual can promote the practice of administering ‘the right drug, at the right time, at the right dose, for the right person’. Pharmaceutical and biopharmaceutical companies are continuously attempting to implement patient-selection diagnostic frameworks in the earlier stages of drug development to provide targeted therapies to the right candidate. This further supports the growth of the companion diagnostics market.
Increasing Demand for Next-generation Sequencing
NGS-based companion diagnostic tests aim to unlock molecular information from each patient’s tumor genome to guide treatment decisions for cancer therapies. Next-generation sequencing detects multiple biomarkers for multiple drug therapies in a shorter time frame as compared to other sequencing techniques. The use of NGS panels for biomarker measurement in one test has the potential to help in the treatment of many different types of cancers. The various technological advancements in NGS also provide market players with an immediate competitive edge over players providing other technologies such as PCR, ICH, and ISH. As a result, major market players are focusing on developing companion diagnostic products based on NGS.
North America Is the Largest Regional Market for Companion Diagnostics
The global companion diagnostics market is segmented into five major regions—North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa. In 2020, North America accounted for the largest share of the companion diagnostics market. The North American companion diagnostics market growth can be attributed to the presence of many leading companion diagnostics vendors & national clinical laboratories, the easy accessibility to technologically advanced devices and instruments, and the highly developed healthcare system in the US and Canada.
Request Sample Report:
https://www.marketsandmarkets.com/requestsampleNew.asp?id=155571681
The prominent players operating in the Companion Diagnostics Market are F. Hoffmann-La Roche Ltd. (Switzerland), Agilent Technologies, Inc. (US), Qiagen N.V. (Germany), Thermo Fisher Scientific, Inc. (US), Abbott Laboratories, Inc. (US), Almac Group (UK), Danaher Corporation (US), Illumina Inc. (US), bioMérieux SA (France), Myriad Genetics, Inc. (US), Sysmex Corporation (Japan), Abnova Corporation (Taiwan), Guardant Health, Inc. (US), ICON Plc (Ireland), BioGenex Laboratories, Inc. (US).