The Nordic regulatory affairs market size was valued at USD 153.2 million in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 7.5% from 2021 to 2028. Changing regulatory landscape, increasing demand for the faster approval process, and economic and competitive pressures are some of the key factors expected to drive the market. The entry of life sciences companies in the Nordic markets, especially in countries such as Denmark and Sweden, for R&D collaborations and the evolution of new areas, such as orphan drugs, biosimilars, advanced therapy medicinal products (ATMPs), and personalized medicine, are further anticipated to contribute to the market growth.
At present, the healthcare industry is focused not only on the development of blockbuster therapies for the treatment of various diseases but also on targeted gene therapies, especially drugs and precision medicine, which help to treat specific diseases and disorders. Some of these products are also being combined with medical devices to enhance the quality of drug delivery and patient monitoring or adherence, thus increasing the complexity of defining the regulatory strategy and pathway to market.
To identify the key drivers and the competitors’ insights, Request a Sample @:
https://www.grandviewresearch.com/industry-analysis/nordic-regulatory-affairs-market/request/rs1
For instance, the development and use of biosimilars continue to grow in the Nordic region. Countries, especially Sweden and Norway, are known to hold leadership positions in crafting policies that facilitate the production of biosimilars. Thus, for faster approval of their biosimilar drugs, companies have to rely on clearance from regulatory bodies, thus creating demand for services in the Nordic countries.
Key Player Mentioned:
- Pharma Assist Sweden AB
- GenPact Ltd.
- ICON plc
- Freyr
- Global Pharma Consultancy AB
- Regsmart Lifesciences AB
- PRA Health Sciences
- Charles River Laboratories International, Inc.
- Parexel International Corporation, Inc.
- Accell Clinical Research LLC
Product Segment Analysis:
- Preclinical
- Clinical Studies
- PMA
The sudden spread of the COVID-19 pandemic affected the ongoing clinical trials at the global level. The impact was also visible in the Nordic countries, such as Norway, Sweden, and Denmark. In Norway, a temporary halt of patient recruitment for new clinical trials was seen. Adherence to new mandates released by each country’s government agency has further created challenges to continue ongoing clinical trials.
Regional Segment Analysis: North America, Europe, Asia Pacific, Latin America, and Mideast and Africa.
Access Press Release: https://www.grandviewresearch.com/press-release/nordic-regulatory-affairs-market-analysis
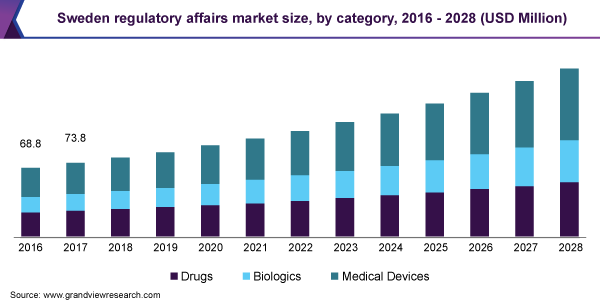
In view of this, in 2018, Genomic Medicine Sweden (GMS) was launched for the development of precision medicine in Sweden. It aimed at implementing precision medicine into the clinical trial settings in the country. In June 2020, Vinnova, an innovation agency, invested USD 4.4 million to promote R&D and collaborations in the field of precision medicine. Precision medicine is a potential area of growth in Sweden. Stockholm, the country’s capital, is home to over 50% of companies focused on the area of precision medicine. Many of these companies are actively pursuing international collaboration and partnerships.
Why Purchase this Report?
- A strong research methodology was followed to collect data for the report. The data collected in this way are subjected to several quality checks to provide the best quality.
- The report provides a holistic view of the competitive scenario of the Nordic Regulatory Affairs Market.
- The latest product launches, along with technological changes and developments, are included in the report.
- The data analysis in the report will help you understand the Nordic Regulatory Affairs Market dynamics projected from 2021 to 2028.
- The Report has a vast data store, so it can accommodate your custom needs as well.
About Us:
Grand View Research is an India & U.S. based market research and consulting company, registered in the State of California and headquartered in San Francisco. The company provides syndicated research reports, customized research reports, and consulting services. Grand View Research database is used by the world’s renowned academic institutions and Fortune 500 companies to understand the global and regional business environment. Our database features thousands of statistics and in-depth analysis on 46 industries in 25 major countries worldwide.